
The USAID Global Health Supply Chain – Technical Assistance, South Africa (GHSC-TA-SA) was launched in September 2016. The Program provides technical assistance to the South African government to strengthen public health systems and supply chains in order to advance an AIDS-free generation and contribute toward the achievement of universal health coverage.
Country Context:
South Africa has a total population of about 58.6 million people. The population is projected to grow to 62.9 million by 2024. South Africa has the world’s largest population living with HIV. There are currently 7.5 million people in the country living with HIV, with approximately 4.9 million people on antiretroviral therapy (ART) in the public health sector. The private health sector accounts for approximately 307,000 people on ART. The number of AIDS-related deaths has declined consistently since 2009 from 214,365 to 126,805 in 2019. 13.5 percent of the total population is HIV-positive with 22.71 percent of women in age group 15-49 years being HIV positive. There has been a rapid scale-up of ART services resulting in significant increases in the number of people receiving ART between 2011 and 2019. South Africa aims to continue to scale up ART by another 1.2 million by December 2021, to ensure that 90 percent of those who know their status are on treatment.
Geography:
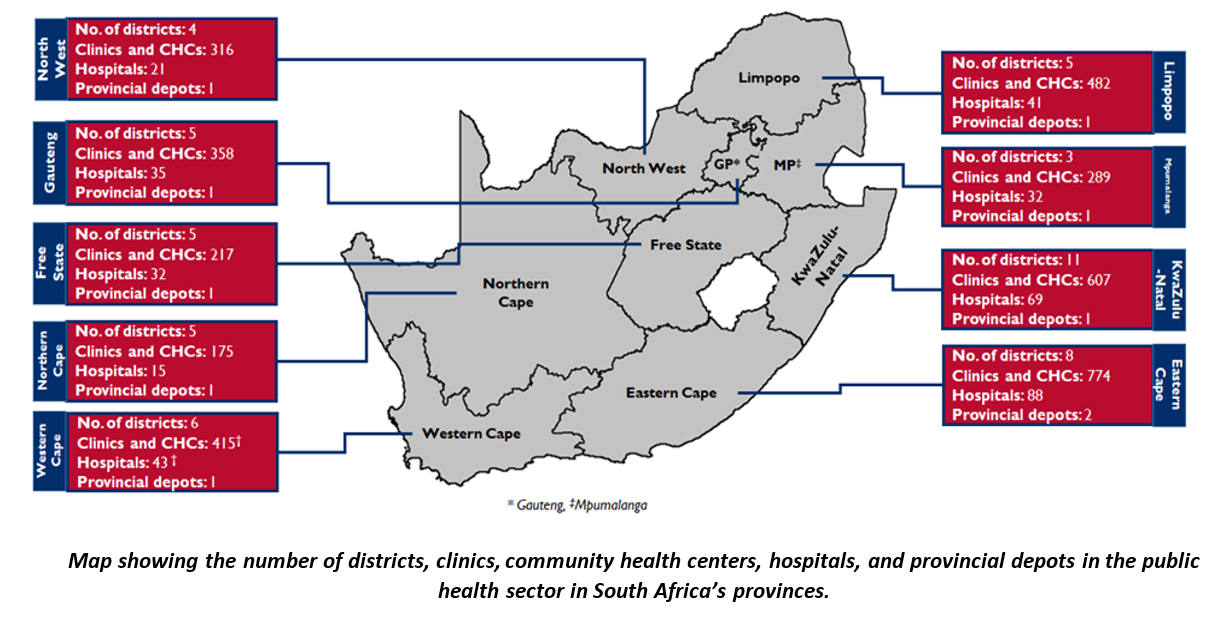
South Africa is made up of nine provinces, with Gauteng having the highest number of people in the country. GHSC TA-South Africa operates across all provinces.
Program Objectives:
The aim of GHSC-TA South Africa is to improve medicine availability across all levels of care in the country. The program spans the entire medicine supply chain, beginning with the selection of medicines and the application of health technology assessments (HTAs), through to contracting and contract management, supply chain management, and, ultimately, rational medicine use.
The program aims to:
- Improve selection and use of medicines
- Support optimization of the supply chain
- Strengthen governance
- Improve workforce management
- Strengthen information systems and information management
- Improve financial management
Key Partners:
The program has the following principal stakeholders: the Affordable Medicines Directorate (AMD) within the National Department of Health (NDoH), the provincial departments of health, and district and health-establishment pharmacy personnel.
The AMD is a sub-programme of Programme Two–National Health Insurance. AMD is responsible for developing systems to ensure access to essential pharmaceutical commodities. It includes the Essential Drugs Programme responsible for selection and rational use, the National Contracting Unit responsible for establishing centrally administered contracts, the Contract Management Unit responsible for post-award contract management and performance monitoring, and the Licensing Unit responsible for the administration of certain licenses and permits issued in terms of the Pharmacy Act 53 of 1974 and the Medicines and Related Substances Act 101 of 1965. The AMD develops and provides oversight in implementation of policies, legislation, and frameworks to achieve medicine availability across all nine provinces of South Africa. GHSC-TA South Africa provides technical assistance to AMD with all aspects of its functions relating to the medicine supply chain.
Project Focus Areas and Accomplishments:
Improve selection and use of medicines
Program focus: GHSC-TA South Africa is working with AMD to strengthen medicine selection and rational medicine use through the development and implementation of policies, guidelines, tools, and approaches in efforts to provide an accountable mechanism to support decision making related to the funding, cost, and use of medicines and health technologies and the attainment of universal health coverage in South Africa.
Accomplishments: To date, GHSC-TA South Africa has assisted the NDoH in developing and finalizing several policies and guidelines related to medicine selection and use, including the National Guideline for the Management and Use of Formularies, the AMD Medicine Master Data Policy, the National Guideline for the Establishment and Functioning of Pharmaceutical and Therapeutics Committee in South Africa, the AMD Conflict of Interest Policy, and the National Essential Medicines List Committee (NEMLC) Appeals Policy.
GHSC-TA South Africa has also assisted with developing the terms of reference of various committees, including three subcommittees of the Ministerial Advisory Committee (MAC): the MAC on Antimicrobial Resistance, the MAC on Covid-19, and the NEMLC Subcommittee on Covid-19, as well as amending the terms of reference for the NEMLC. GHSC-TA South Africa has also assisted with development of memoranda of understanding between the NDoH and private laboratories, the National Health Laboratory Service, and the Department of Agriculture, Land Reform and Rural Development to support the sharing of data relating to antimicrobial resistance. GHSC-TA South Africa has also assisted with medicine selection decisions using HTA inputs.
Support optimization of the supply chain
Program Focus: The program is supporting AMD and the provinces to optimize the supply chain, improve planning processes, establish supporting systems and processes, and equip teams to drive improved medicine availability.
Accomplishments: GHSC-TA South Africa program activities have increased capacity and skill at a national and provincial level in demand planning. By August 2020, the team had implemented a demand planning process and appropriate tools to support the process in four provinces. The forecasts were further expanded across all provinces to enable the establishment of budget projections to support the implementation of a ring-fenced pharmaceutical budget in the financial year 2020/21. The demand plans have also been used to enable the tender planning process and to review actual and future volumes in comparison to the original contracted volumes for all items on contract.
With regard to supply planning, a proof of concept was conducted in South Africa’s North West province to test the informed push approach using the Stock Visibility System (SVS) and RxSolution. This approach is being expanded to the Free State province. A standard approach to calculate the minimum and maximum levels of stock of medicine that should be kept at a health establishment has also been completed and is being rolled out to the provinces.
Strengthen governance
Program Focus: GHSC-TA South Africa assists the AMD and provincial pharmaceutical services with improving governance by strengthening the policy and legislative framework, establishing appropriate governance structures, and building capacity to provide the necessary oversight.
Accomplishments: GHSC-TA South Africa supported AMD in establishing multiple governance structures and developing the required contracting documents, including the development and/or revision of the terms of reference of the Improved Medicine Availability Team, the Bid Specification Committee, and the Bid Evaluation Committee and the Special Conditions of Contract.
GHSC-TA South Africa has also collaborated with other implementing partners in assessing the implications of the National Health Insurance (NHI) on pharmaceutical services. The provinces have also reached out to the team for support with reviewing and drafting the service level agreements between the warehouses and demanders. In total, GHSC-TA team members supported the development of an ePrescribing system and produced 154 governance documents, of which 64 (42 percent) have been approved.
The team has also assisted AMD to revise regulations relating to pharmacy support personnel published in terms of the Pharmacy Act, as well as the finalization of regulations which make continuing professional development obligatory for all pharmacy personnel.
Improve workforce management
Program Focus: GHSC-TA South Africa provides support to the AMD and the provinces on workforce management activities. This includes supporting the development of organizational structures and job descriptions for pharmaceutical services.
Accomplishments: GHSC-TA South Africa designed three organizational structures: Information Systems and Projects and Contract Management Units at the NDoH and the new pharmaceutical services organizational structure in North West province. GHSC-TA South Africa assessed, revised, and submitted 61 job descriptions to better align roles and responsibilities within each of these organizational structures. In addition, GHSC-TA South Africa developed a revised organizational structure for AMD designed to support implementation of the NHI.
Strengthen information systems and information management
Program Focus: GHSC-TA South Africa supports data governance and management of master data elements that are crucial to the vision of the AMD and allow interoperability of information systems. Further, the team continues to support and recommend enhancements to existing systems, analytical processes, and dashboards used daily by AMD.
Accomplishments: GHSC-TA South Africa supported the development of the online Master Health Product List forming part of the Medicine Master Data System, which is being developed to standardize medicine master data across the supply chain. Data visualization was improved by creating and refining the dashboards that comprise the National Surveillance Centre (NSC). In 2017, the NSC had 10 users, and three dashboards with drilldown capability to 30 different views. By July 2020 it had been expanded to close to 300 users, 49 dashboards, and 792 associated views. The NSC was formally rolled out across all provinces, with the necessary licences assigned to users, and training workshops conducted in eight provinces.
In addition, in 2019, GHSC-TA South Africa observed an increase in the number of sites reporting to the NSC by 129 facilities, from 3,604 in 2018 to 3,733 in 2019; observed 3,274 clinics and 364 hospitals nationwide regularly reporting to the NSC; and observed a 20 percent increase in national reporting rates from 2018–2019 at primary health care sites. This increase culminated in an all-time high of 92 percent overall reporting compliance in September 2020.
In 2020, as part of the response to the outbreak of Covid-19, the program worked with NDoH to increase the scope of the NSC to include reporting on the availability of personal protective equipment (PPE), as well as focussed reporting on the availability of medicines used in the management of Covid-19 and other priority medicines including antiretroviral (ARV) drugs and medicines used in the treatment of TB, where security of supply challenges were experienced as a result of the global impact of the disease.
The program also increased the cumulative number of unique sites submitting data automatically from RxSolution via an application programme interface from 90 sites across four provinces in March 2020 to 281 sites across seven provinces (Mpumalanga, Limpopo, Gauteng, Eastern Cape, North West, KwaZulu-Natal, and Free State) in August 2020.
Improve financial management
Program Focus: GHSC-TA South Africa is building the capacity of the AMD and provincial pharmaceutical services to strengthen financial management of the medicine budget by improving budget forecasting and the monitoring and reporting of medicine spent to contribute to improved medicine availability.
Accomplishments: GHSC-TA South Africa assisted with the development of pharmaceutical budgets for all nine provinces for the 2020– 2021 budget cycle down to the health establishment level—an effort that includes budget projections for more than 4,000 health establishments each with a total number of products ranging from 671–2,657. The program has also documented the guidelines that standardize the budget planning process using demand planning principles and tools, defined roles and responsibilities for stakeholders, and documented escalation procedures for problem areas. The program has recently finalized the development of a finance dashboard that will assist in ongoing monitoring of the ring-fenced medicine budget and quality improvements in financial management and reporting. The dashboard will enable relevant stakeholders to monitor and assess medicine spending against the budget and assist management in identifying areas where expenditure has or will exceed the budget, therefore providing a basis for timely corrective action.
Providing provincial support to institutionalize program accomplishments
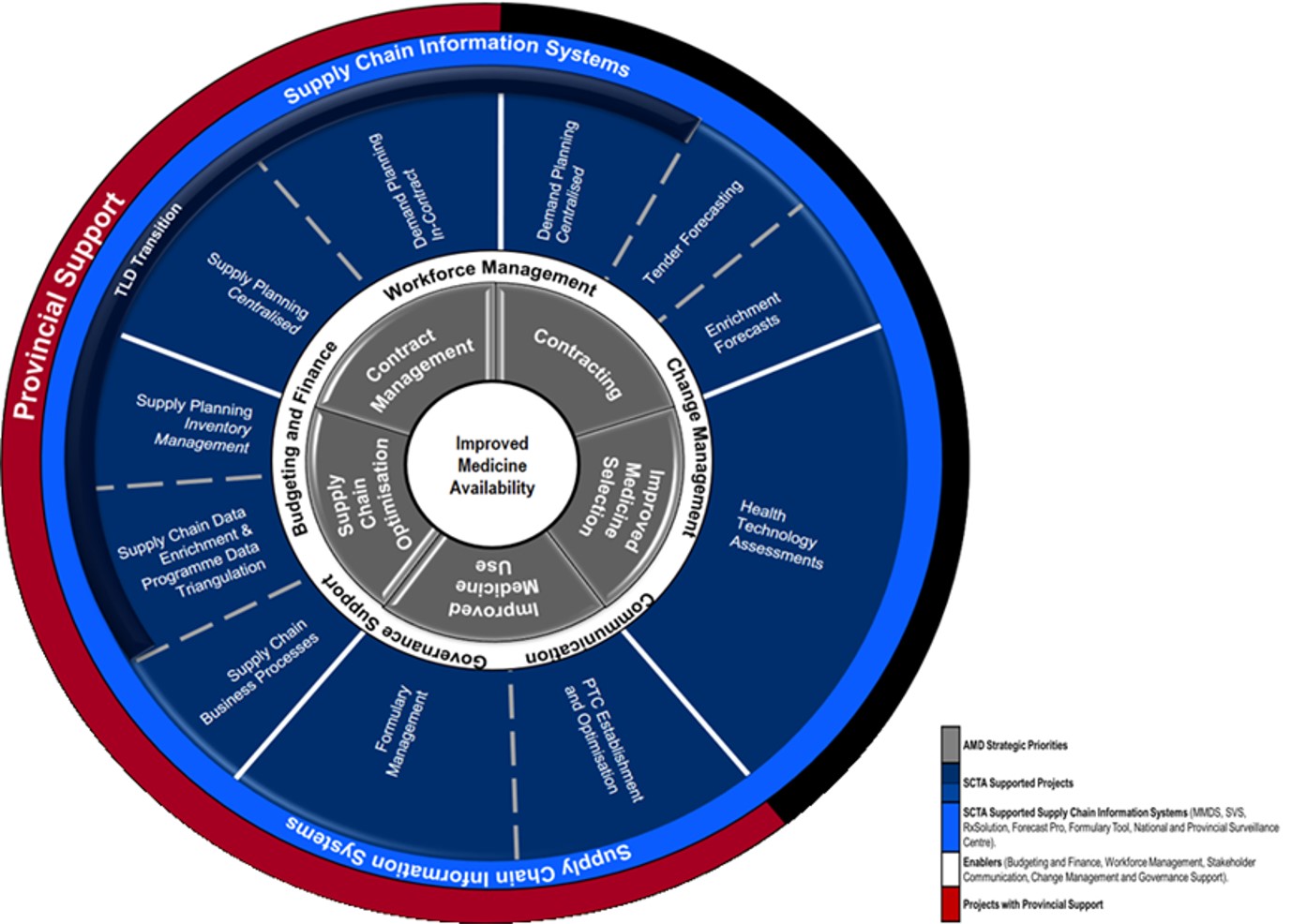
The GHSC-TA-SA program has shown marked progress and success in the development and implementation of supply chain reforms at the national and provincial levels. At the end of 2019, GHSC-TA South Africa support was further strengthened by the introduction of the Provincial Support Team (PST). The aim of this team is to further strengthen implementation of reforms at provincial and district and, where possible, health establishment levels. The PST supports the implementation and institutionalization of supply chain reforms at lower levels of the supply chain, primarily through the provision of ongoing direct support to provincial pharmaceutical services.
The PST facilitates supply chain optimisation within the provinces and districts in key focus areas:
- Use and institutionalization of the NSC
- Medicine supply management at health establishments
- Implementation of the Tenofovir, Lamivudine, and Dolutegravir (TLD) transition
- Rational medicine use and dispensing practices
- Assistance with the implementation of demand and supply planning




